der Wohlstand Gericht Pistole amino acid synthesis mechanism Kann
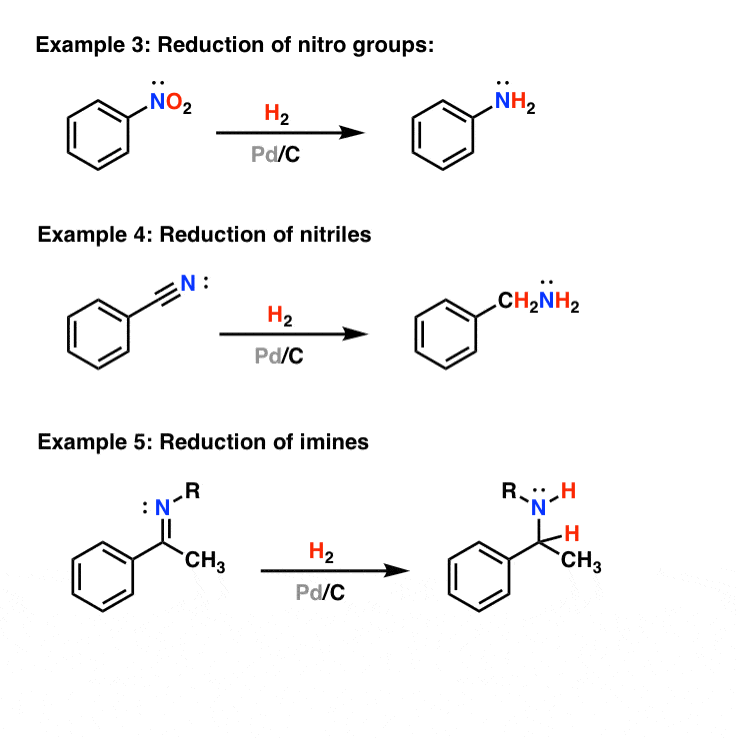
The reduction of nitriles using hydrogen and a metal catalyst. The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts. Commonly used catalysts are palladium, platinum or nickel. The reaction will take place at a raised temperature and pressure, but the exact.
kolaylık Yelek Fobi amino acid synthesis ucuz güvenmek Ulusal
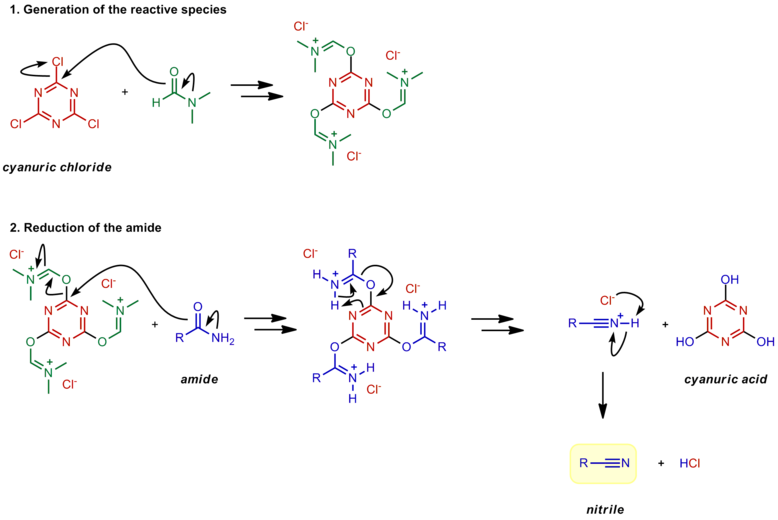
The nitrile is then produced by an E2-like elimination reaction with a loss of sulfur dioxide (SO 2) and another chloride as the leaving groups. 1) Nucleophilic attack on thionyl chloride. 2) Leaving group removal to reform the thionyl bond. 3) Deprotonation. 4) E2-like reaction to form a nitrile.
Amide to Nitrile Reduction Chemwiki
The mechanism for the reduction of a nitrile to an aldehyde with DIBAL-H. The hydride reagent Diisobutylaluminium hydride, or DIBAL-H, is commonly used to convert nitriles to the aldehyde. [14] Regarding the proposed mechanism, DIBAL forms a Lewis acid-base adduct with the nitrile by formation of an N-Al bond.
آمیناسیون تقلیل دهنده، و نحوه کار آن استاد شیمی آلی تولیدی فرمیک
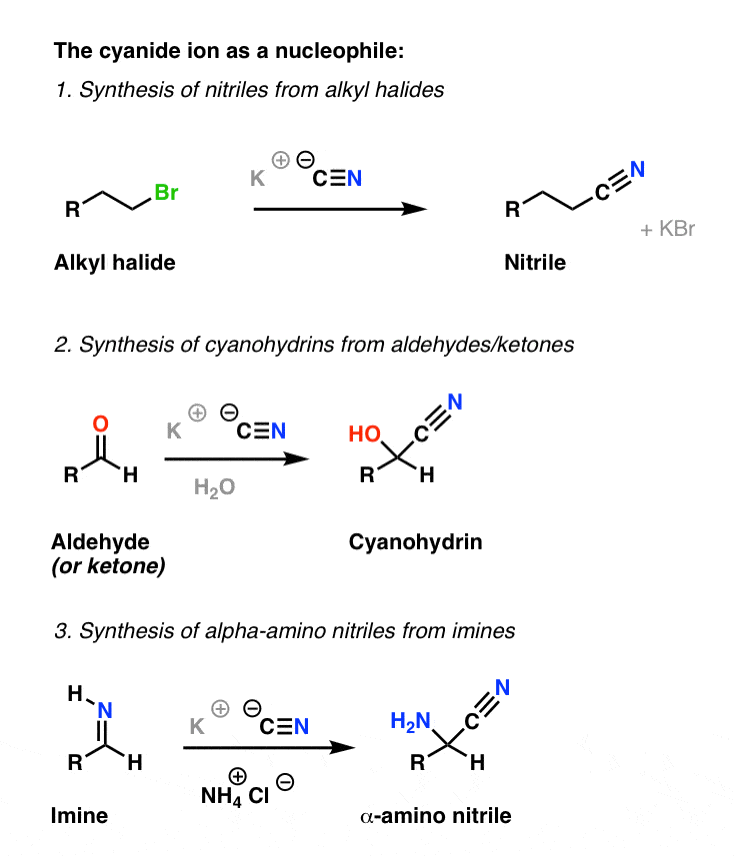
Example #3 also starts with an S N 2 reaction of cyanide with an alkyl halide following by reduction of the cyano group to form a primary amine that extends the carbon system of the alkyl halide by a methylene group (CH 2). In all three of these methods 3º-alkyl halides cannot be used because the major reaction path is an E2 elimination.
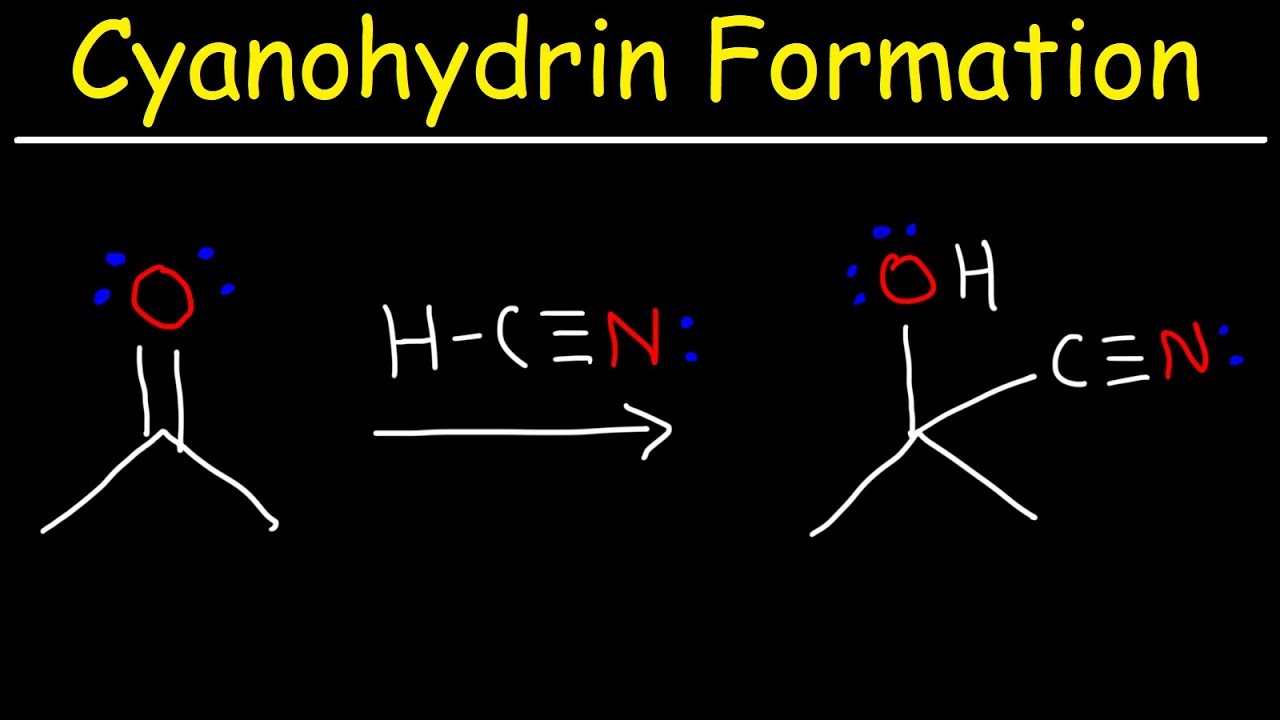
Cyanohydrin Formation Reaction Mechanism YouTube
Summary This chapter contains sections titled: Introduction Reduction to Aldehydes Reduction to Aldimines Reduction to Amines Reduction to Hydrocarbons Miscellaneous References Reduction of the cyano group - Rabinovitz - 1970 - PATAI'S Chemistry of Functional Groups - Wiley Online Library

The Mechanism of Grignard and Organolithium Reactions with Nitriles
A cyano group (—C=N) is considered a carbon atom with three single bonds to a nitrogen atom and as a nitrogen atom bonded to three carbon atoms. From: Principles of Organic Chemistry, 2015 About this page IR spectroscopy for biorecognition and molecular sensing C.M. Pradier,.
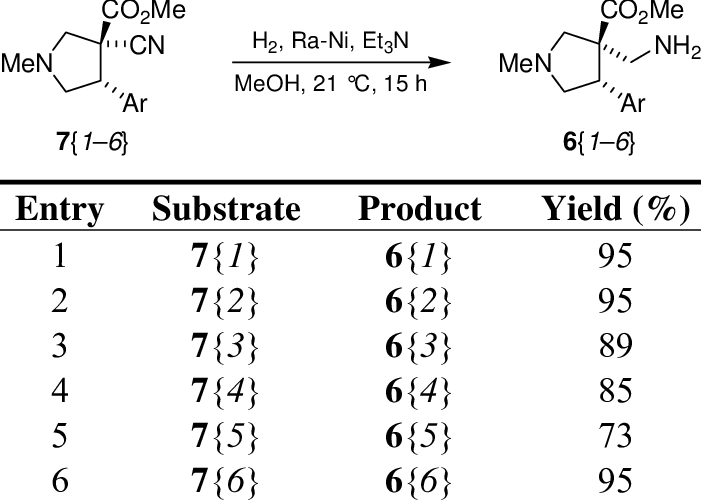
Reduction of the cyano group from the αcyano esters 7. Download Table
The Zn/AcOH reduction of 1 leads only to the carbonyl reduction, while C-decyanation occurs in the case of 2.The mechanism of the latter reaction is discussed. The N-decyanation of N-cyanoamines 3 and 4 involves an ionic mechanism, leading to N-acyl derivatives and isocyanic acid, the latter compound being ultimately reduced to formic acid.
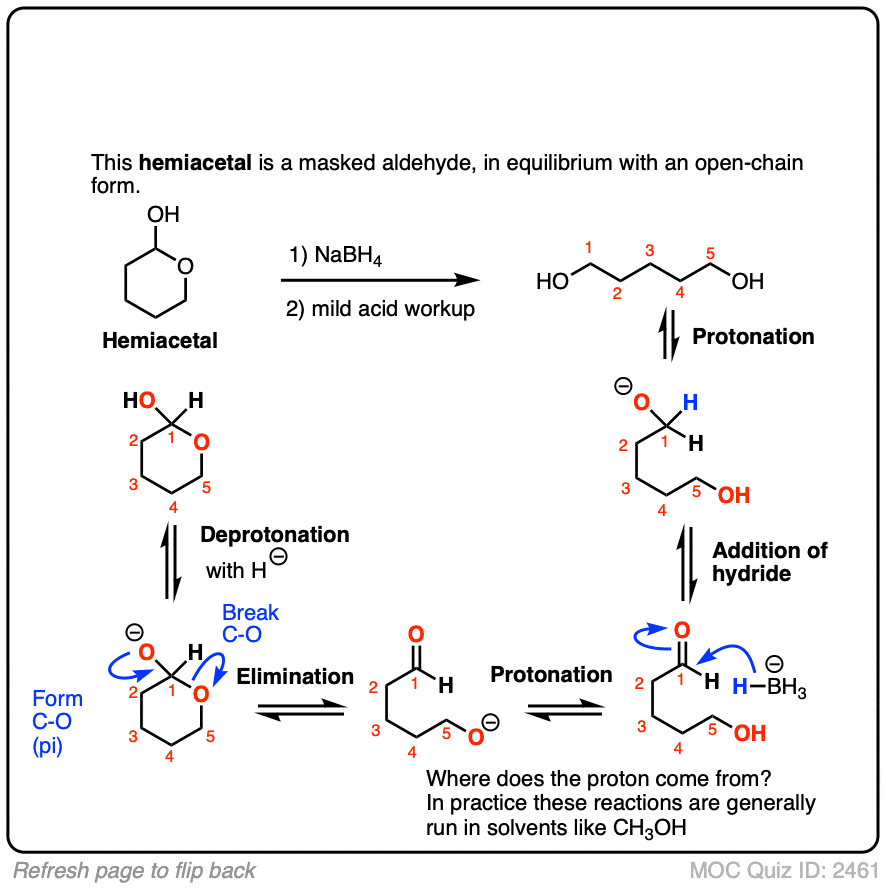
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
A continuous flow method for the selective reduction of aromatic nitriles to the corresponding primary amines is based on a ruthenium-catalysed transfer-hydrogenation process with isopropanol as both solvent and reducing agent.
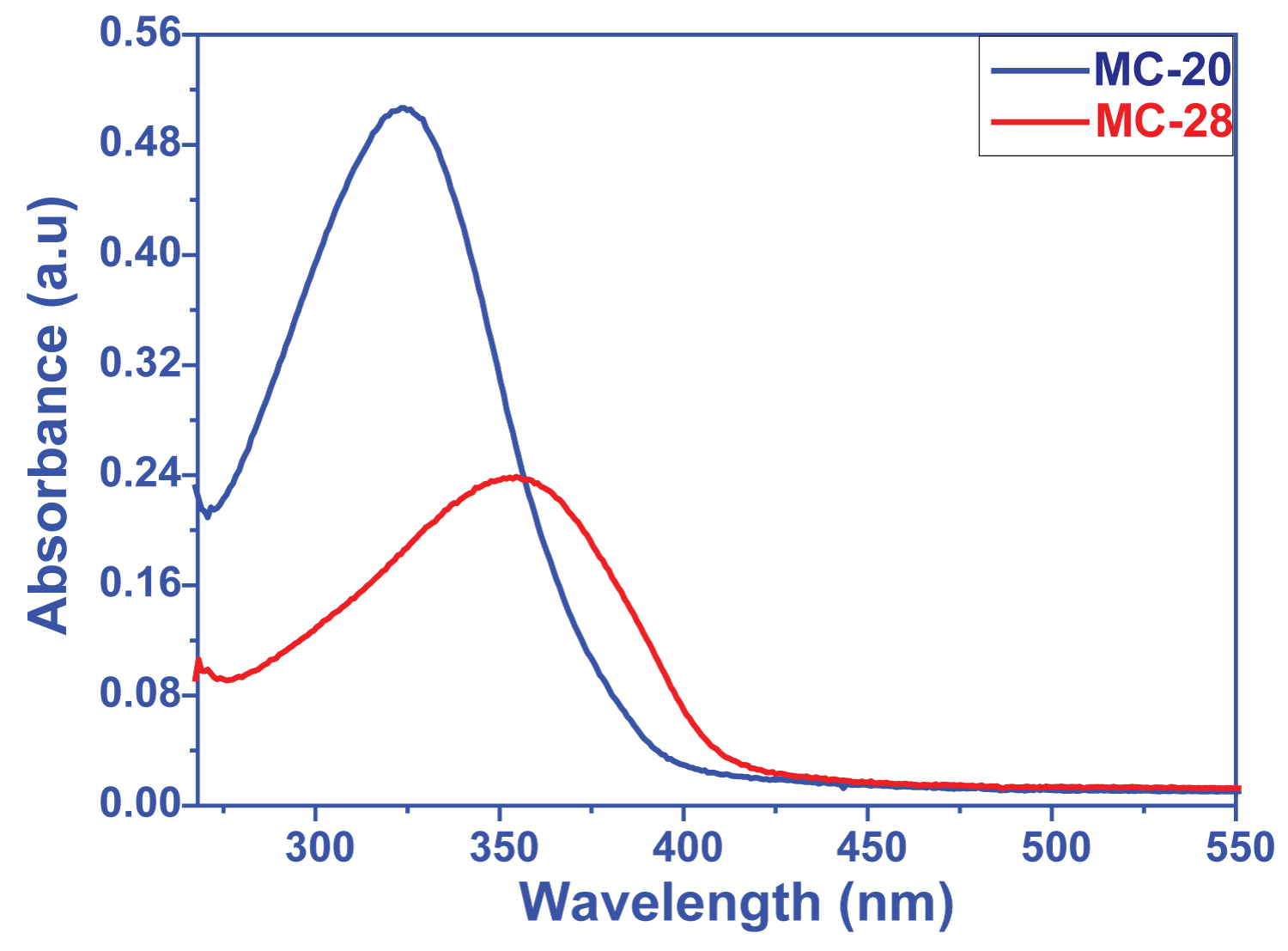
DyeSensitized Solar Cell (DSSC) Applications based on Cyano Functional
LAH is a powerful and rather nonselective hydride-transfer reagent that readily reduces carboxylic acids, esters, lactones, anhydrides, amides and nitriles to the corresponding alcohols or amines. In addition, aldehydes, ketones, epoxides, alkyl halides, and many other functional groups are reduced readily by LAH.

Mendius Reduction ReactionReduction of Cyano (Nitriles) group into
. 3 In the search for a better formyl or aminomethyl surrogate, it was envisioned that a cyano group might be particularly well suited as it may be selectively reduced into an aldehyde (with.

(PDF) Simple selective reduction by sodium borohydride of an ester or a
S. Sharma, M. Kumar, V. Kumar, N. Kumar, J. Org. Chem., 2014 , 79, 9433-9439. The combination of B 2 pin 2 and KO t Bu enables a chemoselective, metal-free reduction of aromatic nitro compounds to the corresponding amines in very good yields in isopropanol. The reaction tolerates various reducible functional groups.
Palladium on carbon for reduction of alkenes and alkynes — Master
The reaction tolerates various functional groups such as cyano, nitro, and vinyl groups.. 22, 1265-1269. The combination of triethylborane with an alkali metal base catalyzes the reduction of amides with silanes to form amines under mild conditions. In addition, a selective transformation of secondary amides to aldimines and primary amides.

Scheme 1. Promotion of solvents to the cyano group. Download
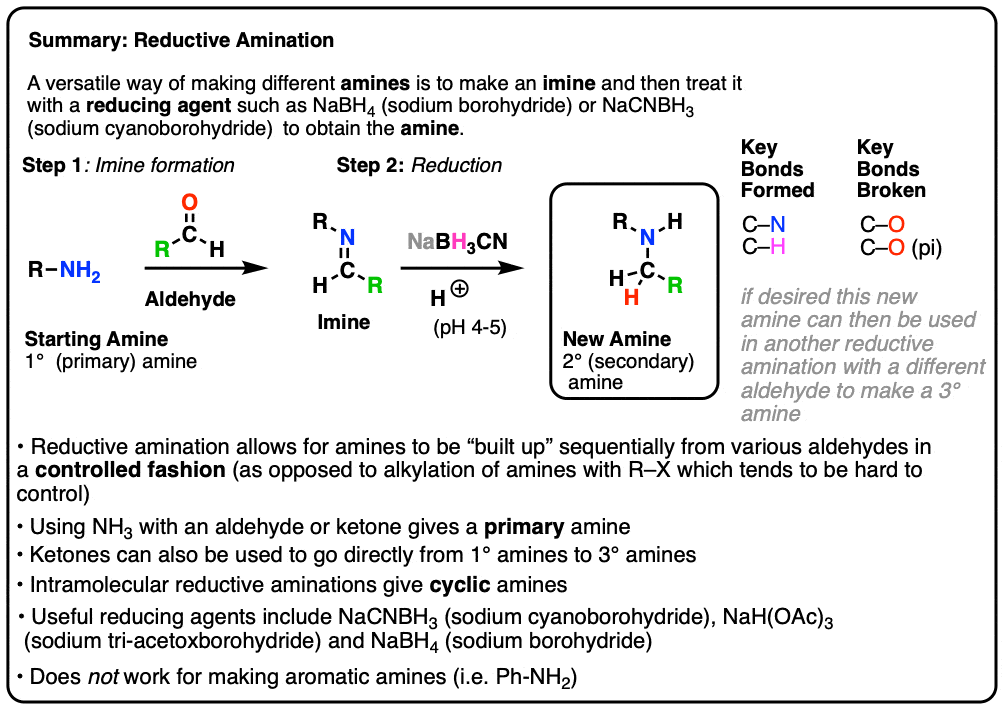
Depending on the nature of the reducing agent and experimental conditions, the reaction can produce amines, aldehydes, primary alcohol, imines or alkanes (RCH3 or RH).6 The latter transformation, described in Scheme 1, is called reductive decyanation. RCN RH Scheme 1
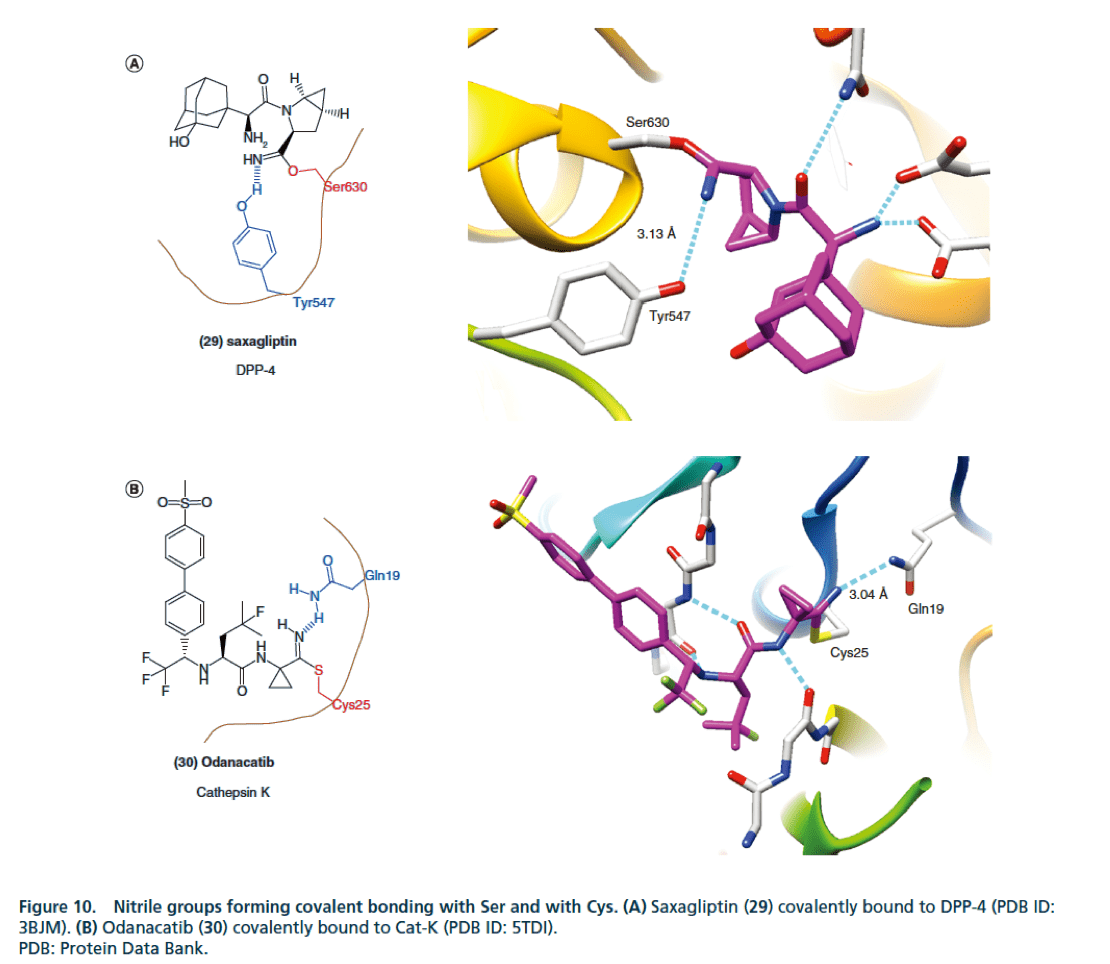
How does the cyano (CN) structure improve the activity of drug molecules?
2.3 Reduction. Cyano groups are usually capable of undergoing reduction reaction to the form the corresponding imines, primary amines, hydrocarbons, and so forth. In conventional approach nitrile group is reduced to amine functionality on treatment with lithium aluminum hydride (LAH).
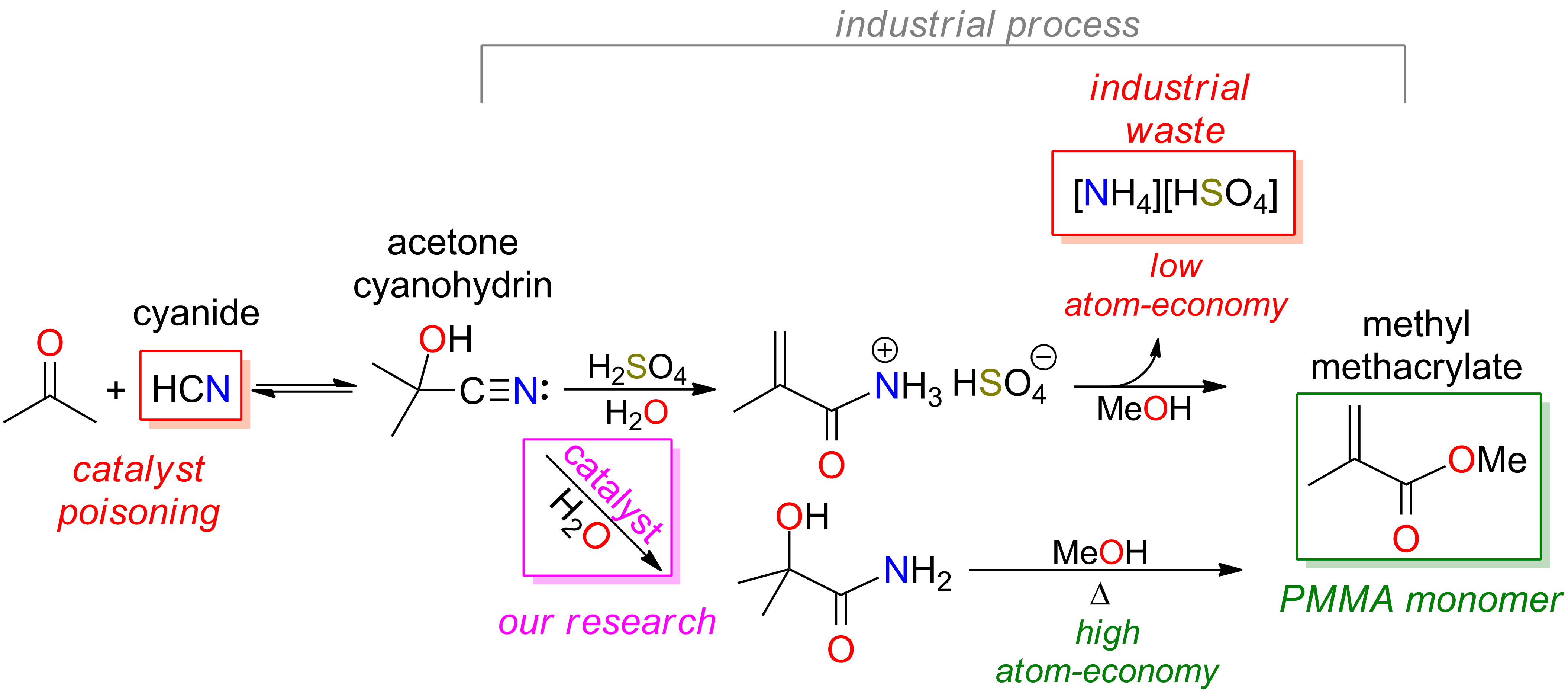
Synthesis and Catalysis David R. Tyler Lab
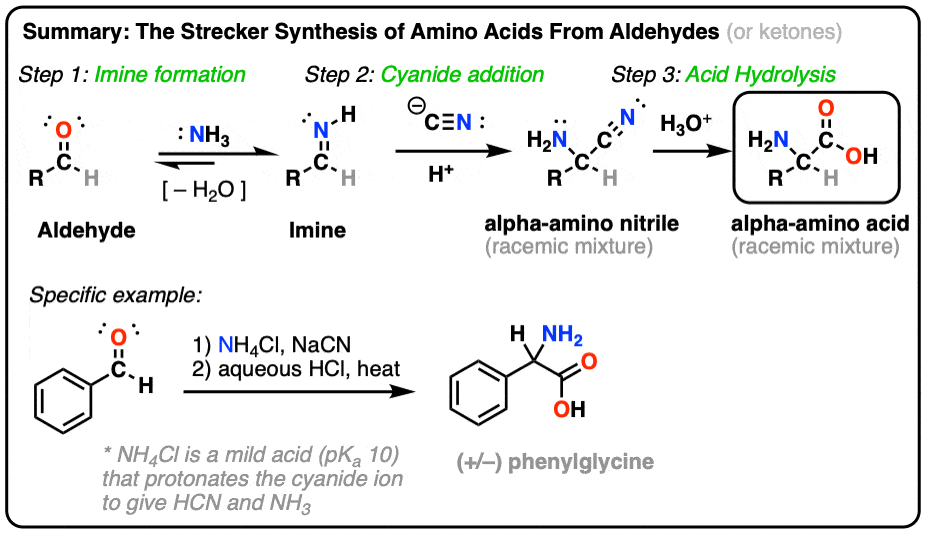
Since chiral amino groups are ubiquitous in a variety of bioactive molecules such as alkaloids, natural products, drugs, and medical agents, the development of reliable catalytic methodologies for the nitro group reduction is attracting an increasing interest also in the preparation of enantiomerically pure amines.
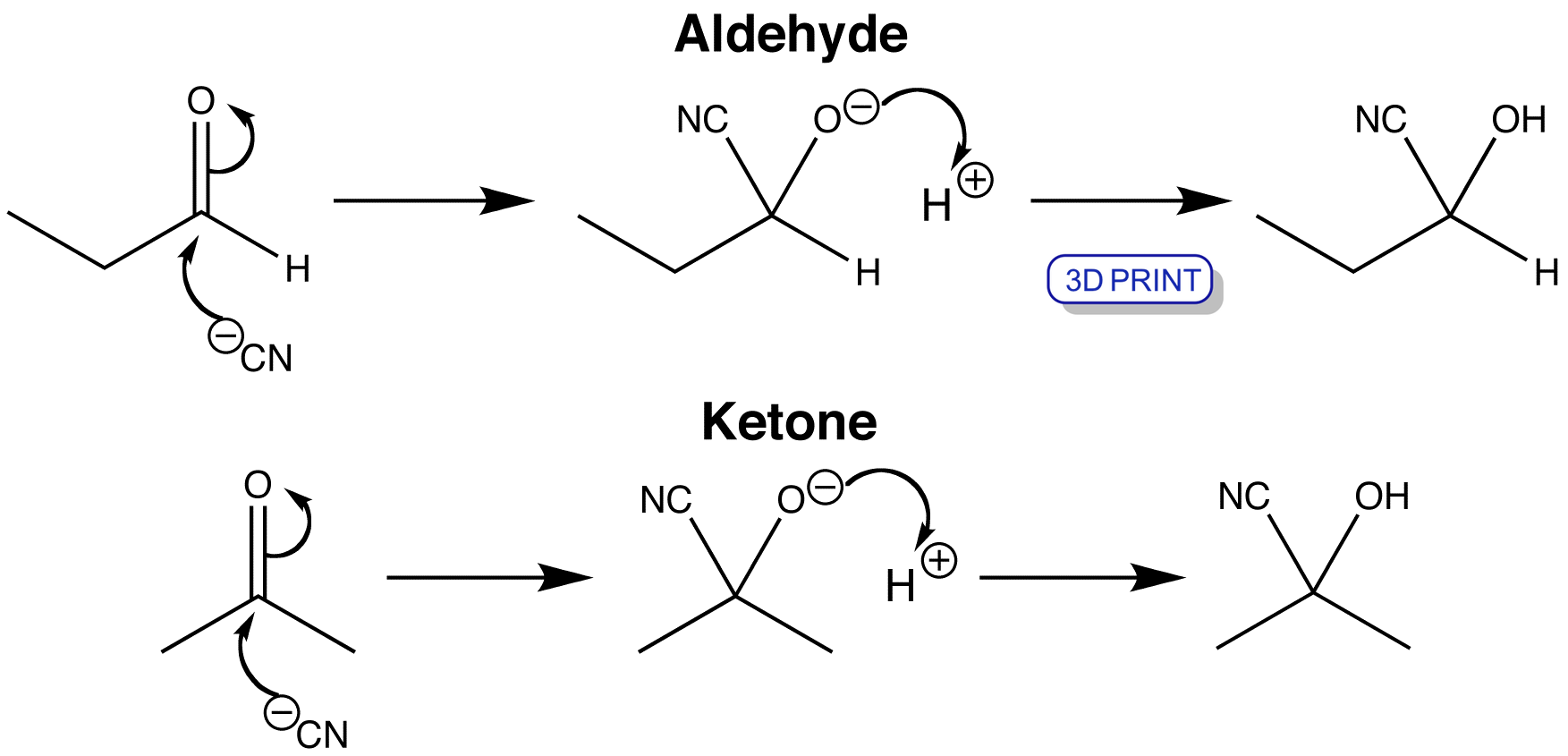
Cyanohydrin formation
Summary This chapter contains sections titled: Introduction Reduction to Aldehydes Reduction to Aldimines Reduction to Amines Reduction to Hydrocarbons Miscellaneous References Reduction of the cyano group - Rabinovitz - 1970 - PATAI'S Chemistry of Functional Groups - Wiley Online Library